Background
Multiple myeloma (MM) is an incurable hematological malignancy of older adults. Autologous stem cell transplant (ASCT) remains a standard of care with multiple retrospective and registry cohort studies demonstrating its efficacy in MM patients including older adults with the disease. Despite this favourable data, there remains wide heterogeneity in the utilization of ASCT, particularly among older adults with MM. We conducted a mixed methods study from the perspective of both oncologists and older adults with MM to: 1) identify decision making factors that influence ASCT eligibility and 2) to explore any barriers to ASCT utilization.
Methods
We conducted a mixed methods study at two academic centres and two community centres in Ontario, Canada. Older adults with MM (aged 65-75) who were within one year of treatment decision making regarding ASCT were invited to complete a survey from outpatient clinics. Oncologists (both community & academic) were recruited via email. Semi-structured interviews were conducted with all participants who agreed to an interview. Thematic analysis was conducted to identify themes from the transcripts using NVivo (qualitative analytical software). The initial 3 transcripts were independently coded by two investigators, to develop a codebook. Any discrepancies were resolved using consensual validation. Once consensus was reached, the codes were then applied to the rest of the transcripts by one coder. A convergent parallel approach was used in combining the results of the qualitative and quantitative sections of the study.
Results
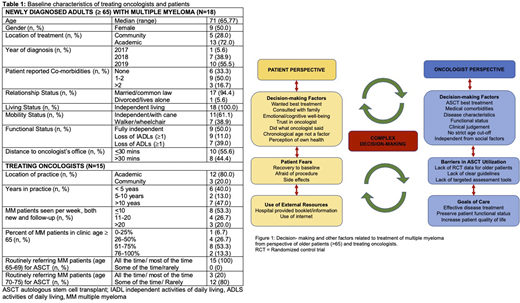
A total of 15 oncologists and 18 patients with MM completed the surveys. Baseline patient and oncologist characteristics are listed in Table 1. The majority of patients were offered an ASCT (78%) and among those offered, 79% went ahead with ASCT. Most patients were happy with the decision to either go ahead or refuse the transplant as indicated by a low decisional regret score (median of 5 and IQR of 0-19 out of 100, with a lower score indicating less regret with the decision). With regards to oncologists, 80% stated they were aware of geriatric tools to help with treatment risk stratification; however, the majority (75%) used none of these tools and relied on the 'eye-ball' test for decision making.
Nine oncologists and 9 patients completed the semi-structured interview. Summarized themes identified are shown in Figure 1. From the perspective of patients, factors that most affected ASCT decision making were: strong trusting relationship with their oncologist (n=9), family support (n=9) and wanting the best treatment available (n=6). Top reasons to refuse ASCT were: fear of not recovering to baseline (n=2) and prolonged hospital stay (n=2). Oncologists identified using their clinical judgement (n=7), the belief that transplant was the best option (n=7) and lack of medical comorbidities (n=8), as the most important factors when recommending treatment. The lack of high quality randomized controlled trial data (n=9), local guidelines (n=5) and targeted assessment tools (n=7) were identified as barriers to ASCT. Notably, both patients (n=7) and oncologists (n=7) felt that ASCT decision making should not rely on chronological age alone. The findings of the qualitative and quantitative parts of the study concurred with each other and showed similar patterns.
Conclusion
To our knowledge, our study is the first to analyze contextual factors from the perspective of oncologists and older adults with MM that influence ASCT decision making and utilization. Despite guidelines supporting ASCT efficacy and safety among older adults with MM, our results demonstrate that the decision to undergo ASCT in older adults with MM is complex and variable both from the perspective of the patient and oncologist. Future incorporation of patient decision aids in parallel with enrollment of older adults in ASCT clinical studies and targeted geriatric assessments tools may provide an opportunity to enhance shared decision making and local guideline developments.
McCurdy:Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Sanofi: Honoraria. Wildes:Carevive Systems: Consultancy; Janssen: Research Funding; Seattle Genetics: Consultancy. Mian:Sanofi: Consultancy; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene: Consultancy; Takeda: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.